PROVEN RESULTS.
PROVEN RELIEF.
Through various clinical trials, OPZELURA has been studied and proven to help clear the visible signs of eczema in people ages 2 and up with mild to moderate eczema and a range of skin tones.
OPZELURA was first studied for 8 weeks in two clinical trials of more than 1200 people ages 12 and older. Later, OPZELURA was studied in an 8-week clinical trial of 330 children ages 2-11.
Results may vary. See the results:
SKIN CLEARANCE
In clinical studies, MORE THAN HALF OF PATIENTS
achieved clear or almost clear skin at 8 weeks
In patients 2-11: 57% achieved results compared to 11% not using OPZELURA
In patients 12+: 54% achieved results compared to 15% not using OPZELURA. Similar results were seen in another clinical study of patients 12+
AT 1 YEAR OF AS-NEEDED USE
After the 8-week studies ended, patients were given the choice to keep using OPZELURA for up to one year as needed when eczema signs and symptoms returned. This was a less rigorous part of the study where patients and their physicians knew they were using an active treatment, which could have affected the results.

PATIENTS STILL USING OPZELURA HAD
CLEAR OR ALMOST CLEAR SKIN


OPZELURA was applied twice daily for 8 weeks on areas where patients had eczema, including sensitive areas such as the face. OPZELURA was used on affected areas, on up to 20% of body surface area. For the 44-week extension period, OPZELURA was applied as needed, twice daily.
OPZELURA is for use on the skin only. Do not use in your eyes, mouth, or vagina. Stop using OPZELURA when signs and symptoms (eg, itch, rash, and redness) of eczema resolve. For patients 12+, do not use more than one 60 gram tube of OPZELURA per week. For patients 2-11, do not use more than one 60 gram tube of OPZELURA per two weeks. Talk to your healthcare provider if you don't see an improvement within 8 weeks.

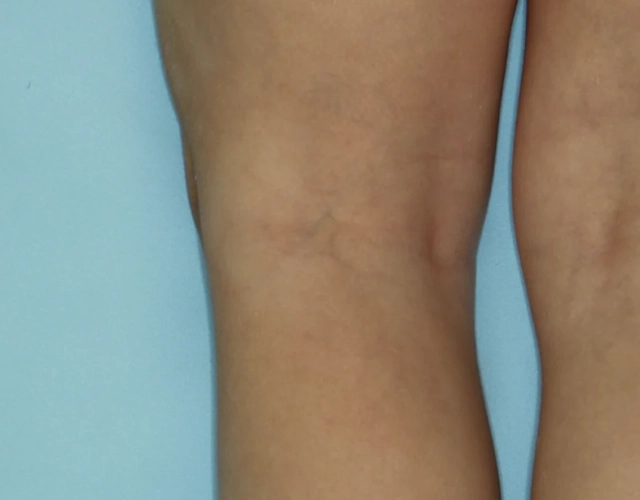
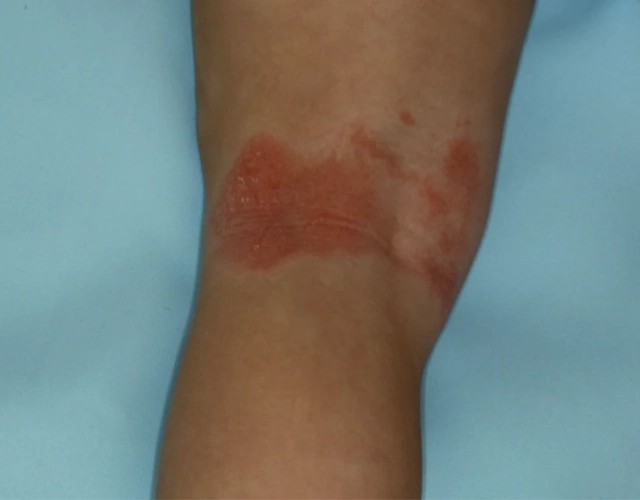
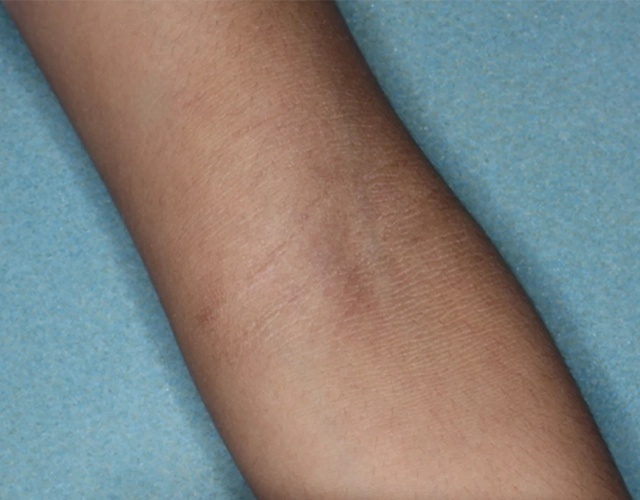
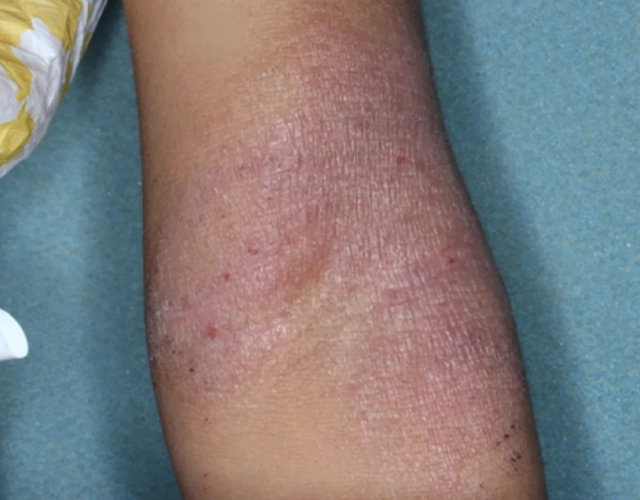
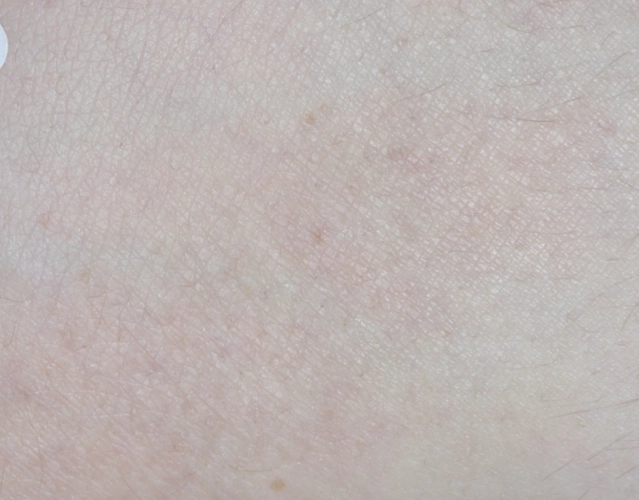
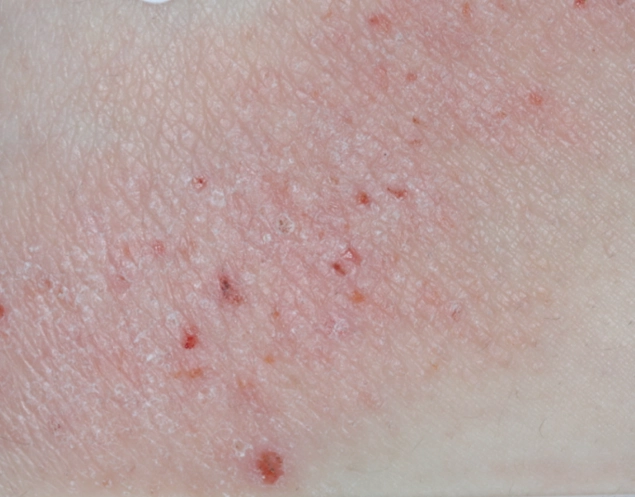
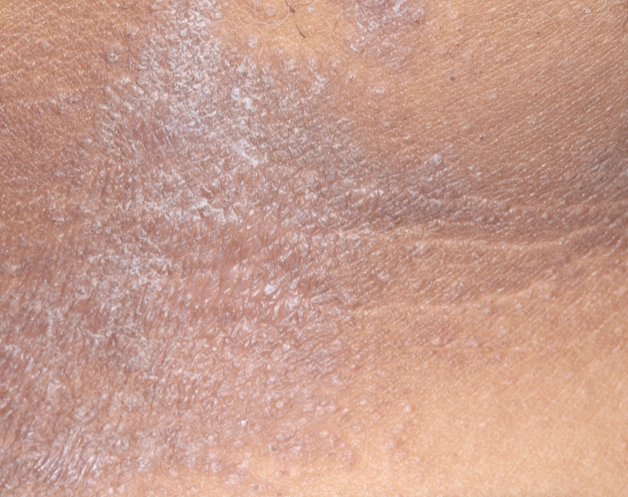
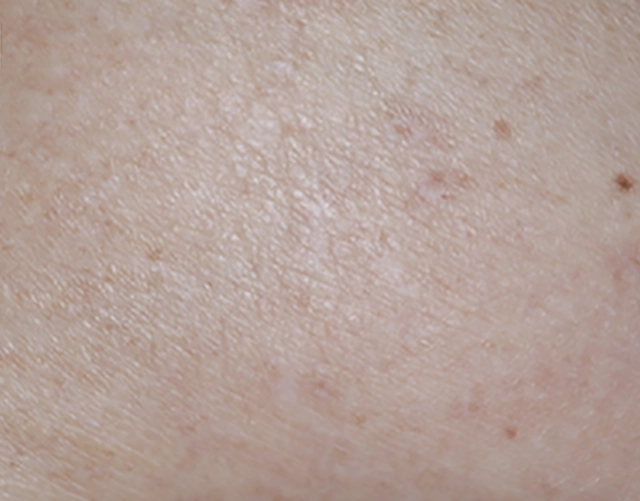
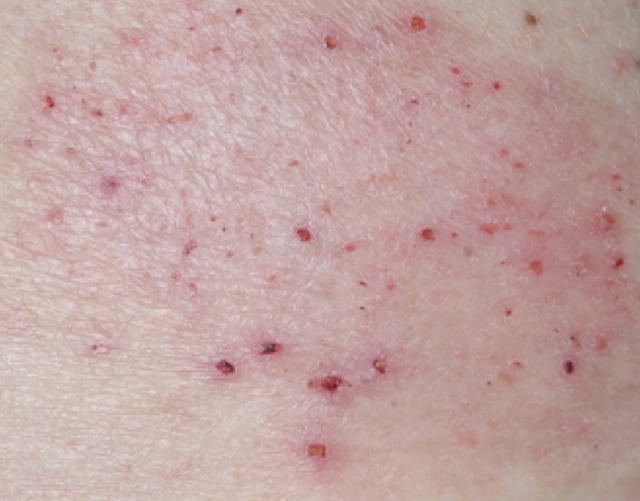
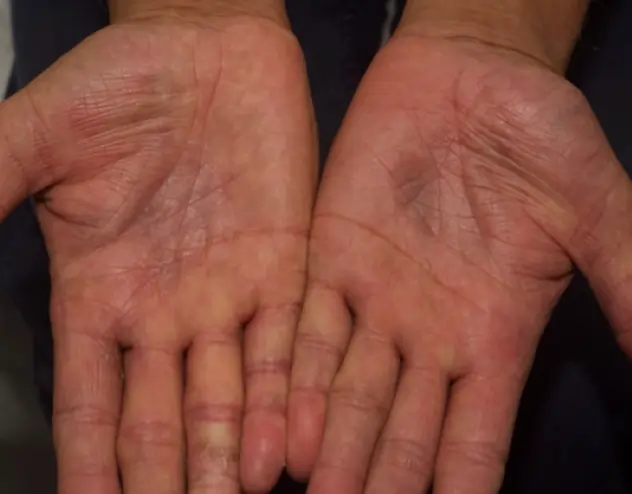
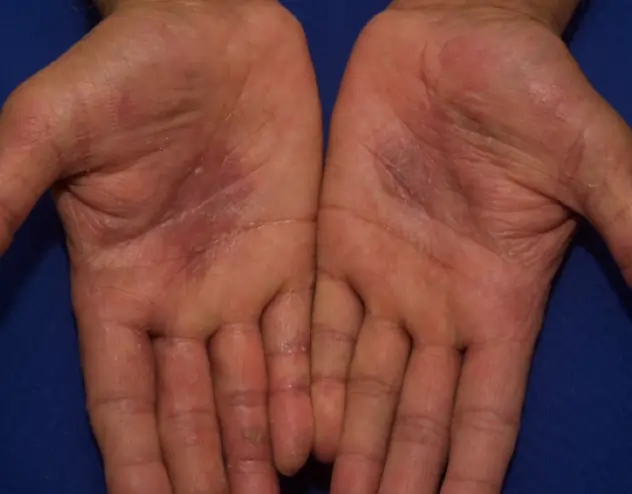
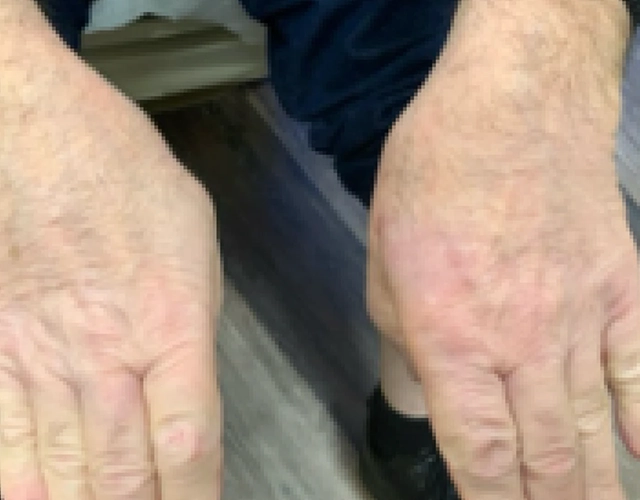
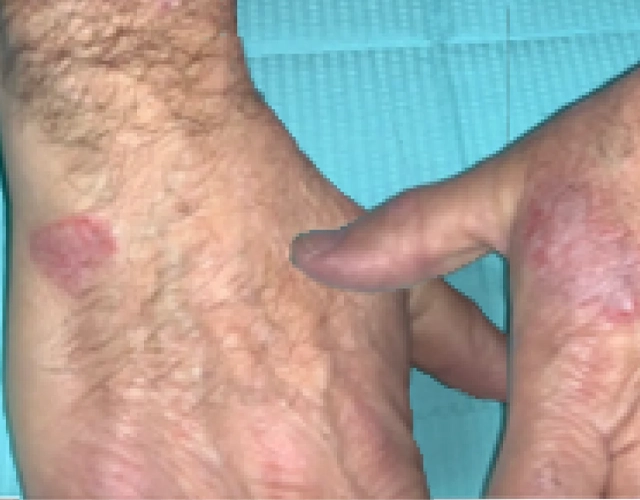
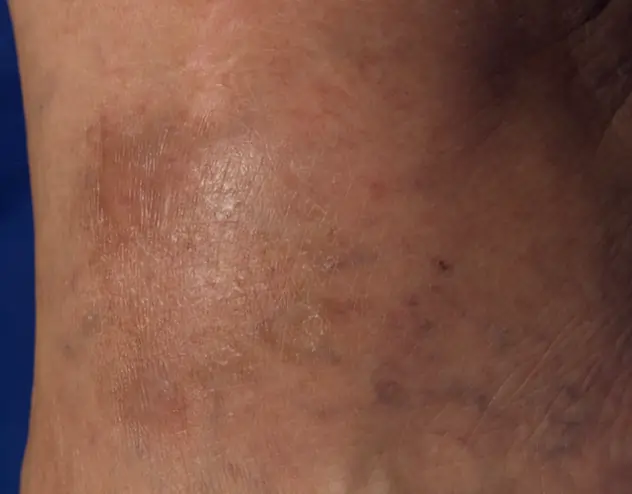
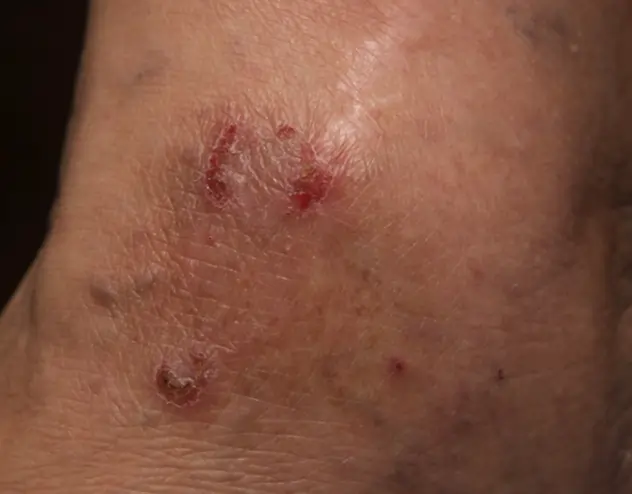
REAL OPZELURA RESULTS
The following photos are a range of patients who used OPZELURA for their mild to moderate eczema, before and after treatment. Since life affects everything, there may be other factors influencing the real-world patients’ treatment results.
Individual results may vary.
Clinical Trial Patients
at week 8
Clinical Trial Patients
at week 8
Real-World Patients
OPZELURA was studied in an 8-week double-blind, vehicle-controlled clinical trial. This means that participants received either OPZELURA or just the vehicle—a cream without medication. Participants were not told which they received. 330 children between the ages of 2 and 11 with mild to moderate eczema participated in this trial.
Who participated in the trials?
Gender
54% female, 46% male
Race/ethnicity
- 55% Caucasian
- 32% Black/African American
- 6% Asian
Percentage of body area affected by eczema
3% to 20%. The average affected area was 10.5%.
Severity*
Participants had an eczema severity of 2 (mild) or 3 (moderate) on a scale of 0 to 4 (where 0 represented no eczema and 4 represented severe eczema). 76% of participants had a score of 3 (moderate).
*People with severe eczema were not included in the trial.
OPZELURA was studied in 2 double-blind, vehicle-controlled clinical trials. This means that participants received either OPZELURA or just the vehicle—a cream without medication. Participants were not told which they received. More than 1200 people with mild to moderate eczema participated in these trials for 8 weeks, with 20% of participants ranging in age from 12-17 years old.
Who participated in the trials?
Gender
62% female, 38% male
Race/ethnicity
- 70% Caucasian
- 23% Black/African American
- 4% Asian
Percentage of body area affected by eczema
3% to 20%. The average affected area was 9.8%.
Severity*
Participants had an eczema severity of 2 (mild) or 3 (moderate) on a scale of 0 to 4 (where 0 represented no eczema and 4 represented severe eczema). 75% of participants had a score of 3 (moderate).
*People with severe eczema were not included in the trial.